Liquid Biopsy for Early Cancer Detection
Our new cancer blood test gives you the earliest possible cancer detection and most accurate treatment monitoring.
Why Choose LBL: Revolutionary Liquid Biopsy Test for Cancer
Monitor Cancer Treatment Efficacy with 99+% Accuracy and Have Supreme Confidence in Your Treatment Recommendations!

Precision oncology should not include guessing or hoping – it should include the most accurate diagnosis possible through medical science today. With Liquid Biopsy Labs’ unique, personalized, and precise testing protocol, you’ll have true confidence in your cancer treatment plan. From start to finish, our testing protocol is designed for certainty. And, we can test for almost any mutation.
Most liquid biopsy tests can only effectively be used for discovery. Because of limitations in their design, standard liquid biopsy tests only show if a patient has a mutation in their blood – meaning that, at best, standard liquid biopsy can be used to confirm what has already been identified in any genetic tumor tissue test. In other words, if you choose a targeted treatment based on the results of a standard liquid biopsy test, your ‘targeted’ therapy is actually more like a shot in the dark, or perhaps a ‘best guess’. You might get it right, but you may also be completely wrong.
After nine years of researching many different liquid biopsy tests from around the world – and exploring combinations of additional diagnostics – research scientist Alexander Rolland (co-founder of Liquid Biopsy Labs), has designed a test that can confidently be used for both discovery and monitoring how well a treatment is working. Our unique, patent-pending test quantitatively measures the amount of a tumor-causing mutation in the blood.
Read more to discover how Liquid Biopsy Labs’ revolutionary liquid biopsy test can show if your patient’s tumor is growing, slowing, or gone – with 99+% accuracy!
The first liquid biopsy – in the world – to test for an almost INFINITE number of mutations.
Why Choose LBL: Revolutionary Liquid Biopsy Test for Cancer
LBL Procedure
Learn about our unique, patent-pending liquid biopsy process! Explore more of LBL’s procedure, step by step, by clicking on the link below.
How LBL’s Tests Differ From Other Standard Liquid Biopsies
Our tests are unique in 4 fundamental ways compared to other companies:
Our Tests Actually Show if Your Cancer is Still Growing or Not
The most important identifier of how well your cancer treatment is working is not the number of dead cancer cells in your blood (which is the measure used by standard liquid biopsy tests). In fact, that commonly used measurement tells you very little about how well your cancer care is working. Think about it. Just because some cancer cells are dying, doesn’t mean your body isn’t producing even more of them.
In reality, in order to have confidence that your treatment plan is working as well as hoped, the information you need is this: the measurement of how many new cells, carrying the cancerous mutation, are still being produced by the body.
Liquid Biopsy Labs is the only liquid biopsy company in the world today to prioritize the monitoring of this crucial indicator of treatment success. We use a rigorous, proprietary method of identifying and isolating exosomal DNA.
Our Tests Provide 99+% Accuracy
 Our analysis is based entirely on exosomal DNA and, therefore, is indicative of metastatic process. We use positive and negative controls to ensure that our information is accurate. This is also unheard of in standard liquid biopsy testing. And it means that, with 99+% confidence, you can tell if the cancer is growing or not.
Our analysis is based entirely on exosomal DNA and, therefore, is indicative of metastatic process. We use positive and negative controls to ensure that our information is accurate. This is also unheard of in standard liquid biopsy testing. And it means that, with 99+% confidence, you can tell if the cancer is growing or not.
And, with LBL tests, you can see any new tumor growth more than 8 months before tumors would be visible through even the most advanced imaging diagnostics, such as PET/CT.
Our Tests Make NO Assumptions
Our test includes a critical factor: verification of the mutation. And while this may sound obvious, it’s a crucial element that other liquid biopsy tests have ignored.
This one error on the part of typical liquid biopsy testing services contributes to a) many patients receiving unhelpful treatments; and b) the medical science world having a confused perspective on the true benefits of liquid biopsy for cancer treatment monitoring.
Other liquid biopsy tests simply tell you whether a mutation is present or not – which tells you nothing about what will work for treatment.
Liquid Biopsy Labs tests, on the other hand, show you whether or not a mutation is causative or problematic. So you don’t just target a mutation because it showed up on some genetic panel, or because it’s commonly associated with a certain type of cancer.
Instead, you have evidence-based confidence that it is a problematic mutation. And, therefore, you don’t waste time targeting the wrong mutation.
For even greater precision, Liquid Biopsy Labs uses a two step identification process:
- We assess that the mutation is present and establish a baseline level of how many new cancerous cells are in the blood sample, and;
- We also confirm the DNA in the normal cells at that same point in the mutation.
Without realizing, most liquid biopsies are based on confounding assumptions about what a ‘normal’ reading should be in that location of the gene. The kits and tools that standard liquid biopsies use to identify a causative mutation are all based on these assumptions of what the original gene contained – rather than assessing, for each individual patient, what is present in that same location naturally for them.
This one element alone can differ greatly from person to person. And, if your liquid biopsy test does not assess each patient’s ‘normal’ (but instead assumes they are all the same), you can see how this would lead to a variety of consequences: completely inaccurate results, less-than-targeted treatment choices, wasted time and money for the medical system – and, most importantly, for the patient.
Liquid Biopsy Labs’ tests ensure that every targeted treatment given is truly matched to each individual patient, and a personalized monitoring test is designed for their unique mutations.
Our Tests are Designed to Give You the Most Rapid and Accurate Treatment Monitoring Tool Available, Worldwide
 Our unique patent-pending test measures, quantitatively, the amount of a tumor-causing mutation in the blood. Therefore, it is an excellent tool for both a) discovery and b) the most precise targeted treatment monitoring.
Our unique patent-pending test measures, quantitatively, the amount of a tumor-causing mutation in the blood. Therefore, it is an excellent tool for both a) discovery and b) the most precise targeted treatment monitoring.
Liquid Biopsy Labs will ensure that you have the most accurate targets for each patient, leading to significantly higher survival rates and greatly reduced treatment side effects.
The use of our unique Liquid Biopsy test allows you to experience unheard of precision in treatment monitoring – providing cancer patients with the best possible outcome that medical science can offer today.
The future of cancer management in a simple blood test.
Order a Liquid Biopsy Test
Avoid misdiagnosis and incorrect treatment plans
Learn More About Precision Oncology

For individuals with cancer, please visit our sister site, Cancer Treatment Options and Management, for Precision Oncology services designed just for you and your care team. CTOAM will coordinate your precision diagnosis – including your personalized liquid biopsy tests – and develop a targeted therapy plan based on what is truly causing your cancer to grow. Cancer Treatment Options and Management will partner with your oncologist to administer and monitor the very best treatment for your cancer.
See how Liquid Biopsy fits into the Four Pillars of Precision Oncology…
Footnotes
- Winslow, Ron. The Race to Diagnose Cancer with a Simple Blood Test. (2019)







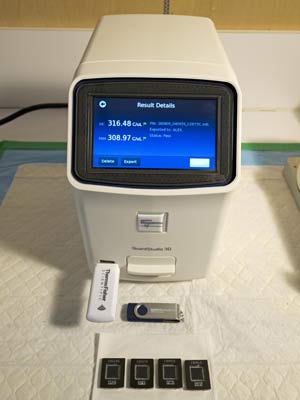
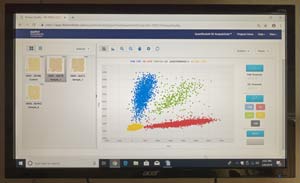
 There are three main ways to assess the accuracy of a test like this: 1. False Negative Rate (Specificity); 2. False Positive Rate (Sensitivity); and 3. Positive Predictive Power (PPV). At LBL, these are usually regarding accuracy.
There are three main ways to assess the accuracy of a test like this: 1. False Negative Rate (Specificity); 2. False Positive Rate (Sensitivity); and 3. Positive Predictive Power (PPV). At LBL, these are usually regarding accuracy. Let’s take an example: Let’s say your tumor tissue test shows a mutated KRAS-G12V gene. G – Glycene – can be coded by GGT, GGC, GGA, and GGG. But let’s say that, when designing their test, ‘Company x’ did a sample study and found that 90% of patients they studied (usually a small sample size, at that) had GGG to start with.
Let’s take an example: Let’s say your tumor tissue test shows a mutated KRAS-G12V gene. G – Glycene – can be coded by GGT, GGC, GGA, and GGG. But let’s say that, when designing their test, ‘Company x’ did a sample study and found that 90% of patients they studied (usually a small sample size, at that) had GGG to start with.